
Example: To make up a 1:3 acetic ethanol solution, you are supposed to mix one unit volume of acetic acid and three unit volumes of ethanol.
Any combination of a water with 4630 us/cm and a water with 720 us/cm will have a conductivity between those two extremes.
#Dilution calculator chemistry serial#
6) Many laboratory protocols require the serial dilution of reagents or compounds. For example, a 1:5 dilution (verbalize as "1 to 5" dilution) entails combining 1 unit volume of solute (the material to be diluted) + 4 unit volumes of the solvent The diluted material must then be thoroughly mixed to achieve the true dilution. dilution factor is the total number of unit volumes in which your material will be dissolved. Gently mix the diluted test sample and follow Steps 5-11 as instructed for counting Fresh Cell products. The final dilution factor is 6 (3 x 2) after 1:1 dilution with 0.4% trypan blue for counting. A 3-fold dilution (20μL sample + 40μL DPBS) will result in a test sample of approximately ≥ 1.87 x 10^6 cells/mL. On adults, for daily use and application for the entire body, users should maintain around 1% dilution of essential oils. Diluting Essential Oils – Dilution Percentages Some aromatherapy experts suggest that for use on children under two years old, only dilutions of. Decreases in equivalent conductance of selected electrolytes with increas ing concentration 8 5. Values of the ratio of specific conductance to conductivity for 0.01 N KG solution and 1 per mil chlorinity seawater 6 4.

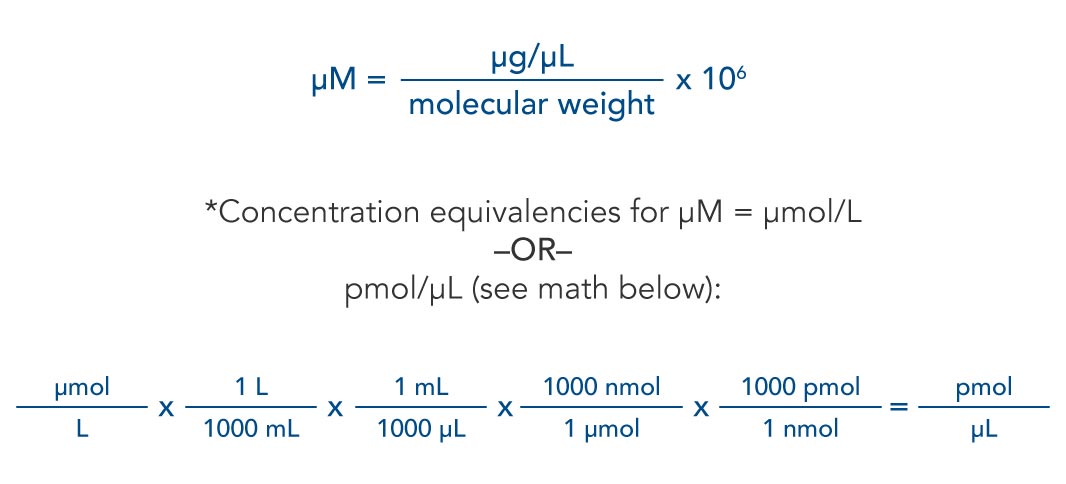
Percentage change in conductivity with temperature for 0.01 N KC1 solu tion and seawater in 1 ☌ increments 5 3. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.


 0 kommentar(er)
0 kommentar(er)
